Executive Summary
Pharma regulatory technology has come a long way from paper chaos to digital efficiency. Today’s strict rules and complex data present both challenges and opportunities for innovation. AI is set to revolutionize compliance by automating tasks, analyzing big data, and predicting issues, making the process smarter, faster, and easier.
Introduction
In Pharma, regulatory compliance isessential for safe and effective drug development. Ensuring every drug meets stringent standards is crucial for patient safety and company credibility. Over the years, regulatory technology has evolved significantly, driven by tech advances and a focus on transparency and accountability. Regulatory processes
used to be plagued by paperwork and manual errors, but as technology evolved, compliance tools improved. Now, AI is set to revolutionize regulatory practices, making them more efficient, accurate, and proactive. This white paper explores the evolution of regulatory technology, the current compliance state, and AI’s transformative potential, ensuring the Pharma industry stays compliant,innovative, and ahead of the curve.
Section 1: Evolution of Pharma Regulatory Technology
The evolution of regulatory tech in Pharma has been remarkable. We’ve moved from drowning in paperwork to embracing efficient digital systems, making compliance faster and more accurate.
1.1 Early Regulatory Practices and Limitations
In the past, regulatory submissions relied heavily on paper. Companies dealt with piles of documents, manually reviewed by regulatory bodies—a slow, error-prone, and costly process that often led to delays and mistakes.
1.2 Key Technological
Advancements Over the Decades The 1980s introduced computers
and Electronic Document Management Systems (EDMS), which replaced paper, making document control and retrieval easier. The 1990s brought the internet, revolutionizing everything. Online submissions and electronic communications with regulatory bodies became possible, cutting down the time and cost of physical submissions.
1.3 Impact on Regulatory Compliance and Pharma R&D
These advancements were transformative. EDMS and online submissions greatly improved document accuracy, cut down review times, and boosted efficiency. The quick retrieval and analysis of regulatory documents enhanced decision-making in R&D, enabling Pharma companies to focus more on innovation and less on administrative tasks.
Section 2: Current State of Regulatory Technology in Pharma
Regulatory technology in Pharma is continually advancing. Today, the industry
leverages advanced software and platforms to meet regulatory demands efficiently.
2.1 Overview of Latest Technologies in Regulatory Compliance
Modern regulatory tech includes a variety of tools designed to simplify compliance. Regulatory Information Management Systems (RIMS) and cloud platforms manage data, provide real-time updates, and facilitate seamless teamwork. Advanced analytics and machine learning enhance the accuracy and efficiency of regulatory submissions.
2.2 Case Studies/Examples of Successful Implementations
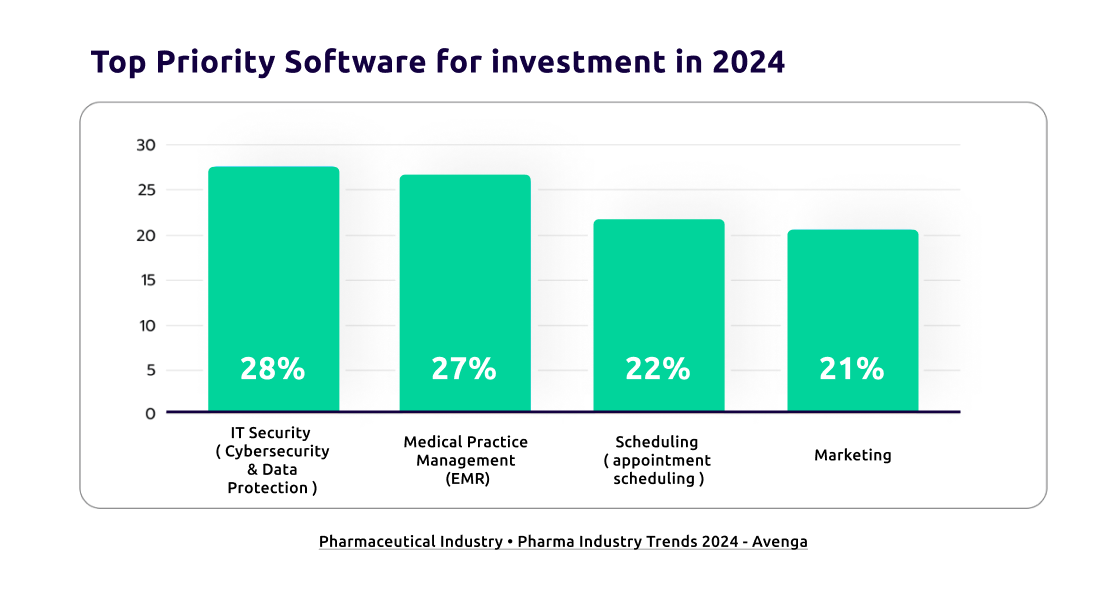
Many Pharma companies have effectively implemented these technologies. For example, one client used a cloud-based RIMS to reduce submission times by 30% and improve data accuracy. Another client utilized business intelligence to predict compliance issues and address them proactively, avoiding delays and extra-legal reviews. According to the “Pharma Industry Trends 2024” report by Avenga, 27% of investments in the pharmaceutical industry will focus on digital transformation through Electronic Medical Record systems (EMS), matching the spend on IT security and infrastructure.
2.3 Benefits Observed: Improved Quality,
Efficiency, and Reliability in R&D The benefits are clear. Enhanced data accuracy and real-time updates ensure consistent and error-free regulatory submissions. Predictive capabilities help identify and resolve compliance issues early, boosting the reliability of R&D processes. Streamlined workflows and faster submissions enable companies to bring new drugs to market more quickly, benefiting patients and healthcare
providers.
Section 3: The Future of Regulatory Compliance with AI
AI is set to revolutionize regulatory compliance in the pharmaceutical industry by making
processes smarter, faster, and more reliable. Here is how AI is transforming the landscape:
3.1 Introduction to AI and Its Relevance to Regulatory Compliance
AI offers significant potential for regulatory compliance. By analyzing large datasets, identifying patterns, and predicting outcomes with unmatched accuracy and speed, AI can automate routine tasks and provide deep insights, vastly improving the efficiency and effectiveness of compliance processes.
3.2 Potential Benefits of AI in Regulatory Technology
Predictive Analytics AI can analyze trends and predict potential compliance risks, allowing companies to address issues before they escalate. This proactive approach can save significant costs and protect the company’s reputation.
Automation Repetitive tasks can be automated with AI. For example, AIdriven audits can scan documents and identify discrepancies in seconds, saving time and reducing human error.
Real-Time Monitoring AI enables continuous compliance monitoring. Instead of periodic checks, AI systems provide real-time oversight, immediately flagging any deviations.
3.3 Examples of AI Applications in Compliance
AI-Driven Audits AI systems can audit regulatory submissions in real time, ensuring every detail is accurate before documents are submitted. This reduces rejection rates and streamlines the approval process.
Automated Documentation
AI can manage and update documentation, ensuring all regulatory requirements are met without manual intervention. This ensures compliance and frees up human resources for more strategic tasks.
Predictive Risk Management
AI can analyze trends and predict potential compliance risks, allowing companies to address issues before they escalate. This proactive approach can save significant costs and protect the company’s reputation.
Section 4: AI Solutions for Staying Compliant
Using AI for regulatory compliance is more than adopting new tech; it’s about integrating it into your processes and culture. Here are some practical solutions and strategies for leveraging AI in compliance:
4.1 Examples of AI Tools and Technologies for Compliance
Regulatory Information Management Systems (RIMS)
AI-powered RIMS can manage large amounts of regulatory data, ensuring all submissions are complete, accurate, and compliant. These systems can also track regulatory changes and update compliance protocols.
Machine Learning Algorithms
These algorithms can analyze large datasets to identify patterns and anomalies. For example, in pharmacovigilance, AI can analyze adverse event reports to detect early signals of potential drug safety issues.
Natural Language Processing (NLP)
NLP can analyze regulatory texts and extract relevant information, making it easier to stay updated with the latest regulatory changes.
4.2 Case Studies of Pharma Companies Successfully Using AI
Pfizer : Pfizer implemented an AI-driven RIMS, reducing submission preparation time by 40% and improving compliance accuracy. This sped up the approval process and minimized the risk of non-compliance penalties.
Johnson & Johnson : Johnson & Johnson used machine learning algorithms to enhance their pharmacovigilance processes. By analyzing large volumes of adverse event data, they identified potential safety issues much earlier, ensuring patient safety and regulatory compliance.
4.3 Strategies for Implementing AI in Regulatory Processes
Start Small : Begin with pilot projects to test AI solutions on specific compliance tasks. This helps you assess their effectiveness and make necessary adjustments before a full-scale implementation.
Collaborate Across Functions : Successful AI integration requires collaboration between IT, regulatory, and business teams. Cross-functional collaboration ensures AI solutions meet specific regulatory compliance needs.
Continuous Learning : AI implementation is an ongoing process. Continuous learning and adaptation are essential. Invest in training your teams to understand and effectively use AI tools.
Section 5: Overcoming Challenges in AI Implementation
Implementing AI in regulatory compliance comes with its challenges. While the potential benefits are significant, several hurdles must be navigated for successful integration.
5.1 Potential Barriers to AI Adoption in Regulatory Compliance
Data Quality and Availability
AI relies heavily on data. Poor quality or unavailable data can limit AI’seffectiveness. Ensuring high-quality,comprehensive data is essential for accurate and meaningful AI insights.
Regulatory Uncertainty
The regulatory environment is constantly changing. New regulations and guidelines can impact AI implementation, creating uncertainty. Companies must stay agile and adapt their AI strategies to comply with evolving regulations.
Cost and Resource Allocation
Implementing AI solutions requires significant investment in technology, infrastructure, and talent. This can be a barrier, especially for smaller companies. Additionally, reallocating resources to support AI initiatives can be challenging.
5.2 Solutions and Best Practices for Overcoming These Challenges
Ensuring Data Quality : Invest in data management practices to ensure your datasets are complete and accurate. This includes data cleansing, normalization, and enrichment processes. Partnering with data providers and using advanced data integration tools can also improve data quality.
Staying Agile in Regulatory Compliance : Maintain a robust compliance monitoring system to keep up with regulatory changes. Engage with regulatory bodies and participate in industry forums to stay informed about new trends and guidelines. Flexibility and adaptability in AI strategies are key.
Cost-effective AI Implementation : Start with pilot projects to demonstrate AI’s value and gain stakeholder support. Use cloud-based AI solutions to reduce infrastructure costs and consider partnerships with AI vendors to leverage their expertise. Investing in employee training can also maximize AI returns.
5.3 The Role of Cross-Functional Collaboration in Successful AI Implementation
Successful AI implementation requires collaboration across various functions within the organization. IT, regulatory, legal, and business teams need to work together to ensure AI solutions meet compliance requirements. Establishing cross-functional teams and fostering a culture of collaboration and innovation can drive successful AI adoption.
Section 6: Efficient Vision – Pioneering Change in Pharma Compliance
At Efficient Vision, we lead the way in technological innovation within the dynamic Pharma regulatory landscape. Our commitment to excellence and adaptability makes us a trusted partner in the industry.
6.1 Our Commitment to Innovation
Efficient Vision is a leader in integrating cutting-edge technologies into regulatory processes. We focus on innovation to ensure our clients stay ahead of the curve, leveraging the latest advancements to streamline compliance and enhance operational efficiency.
6.2 Adapting to Industry Changes
Our culture at Efficient Vision is built on adaptability, collaboration, and continuous learning. We understand the dynamic nature of the Pharma industry and its evolving regulatory requirements. Our team stays informed about industry changes and proactively adapts our strategies to meet new challenges. This proactive approach ensures our clients remain compliant and competitive.
Final Thoughts
The evolution of Pharma regulatory technology has transitioned us from paper-based processes to advanced digital platforms, enhancing efficiency and accuracy in R&D. Today, AI is poised to revolutionize regulatory compliance with predictive analytics, automation, and real time monitoring.
AI’s potential to transform regulatory processes is vast. Industry leaders like Pfizer and Johnson & Johnson have shown that AI can significantly improve compliance, reduce costs, and speed up the time-to-market for new drugs. However, integrating AI presents challenges such as ensuring data quality, managing regulatory uncertainties, and handling implementation costs. Overcoming these hurdles requires strategic planning, cross-functional collaboration, and a commitment to continuous learning.
Embracing AI for a New Era in Pharma Regulatory Compliance
Effvision Business Solutions is at the forefront of this transformation. Our dedication to innovation, adaptability, and scalability makes us a trusted partner in navigating regulatory compliance complexities. Our successful projects demonstrate our ability to deliver AI-driven solutions that enhance efficiency and compliance accuracy.
The future of Pharma regulatory compliance is promising with AI. Industry leaders must embrace this change to drive innovation and operational excellence. By leveraging AI, we can create a more efficient, accurate, and proactive regulatory landscape, ensuring the Pharma industry continues to thrive and innovate.
Let’s seize this opportunity to shape the future of regulatory compliance together. With AI, we can achieve unprecedented levels of operational excellence and ensure patient safety, propelling the Pharma industry to new heights.